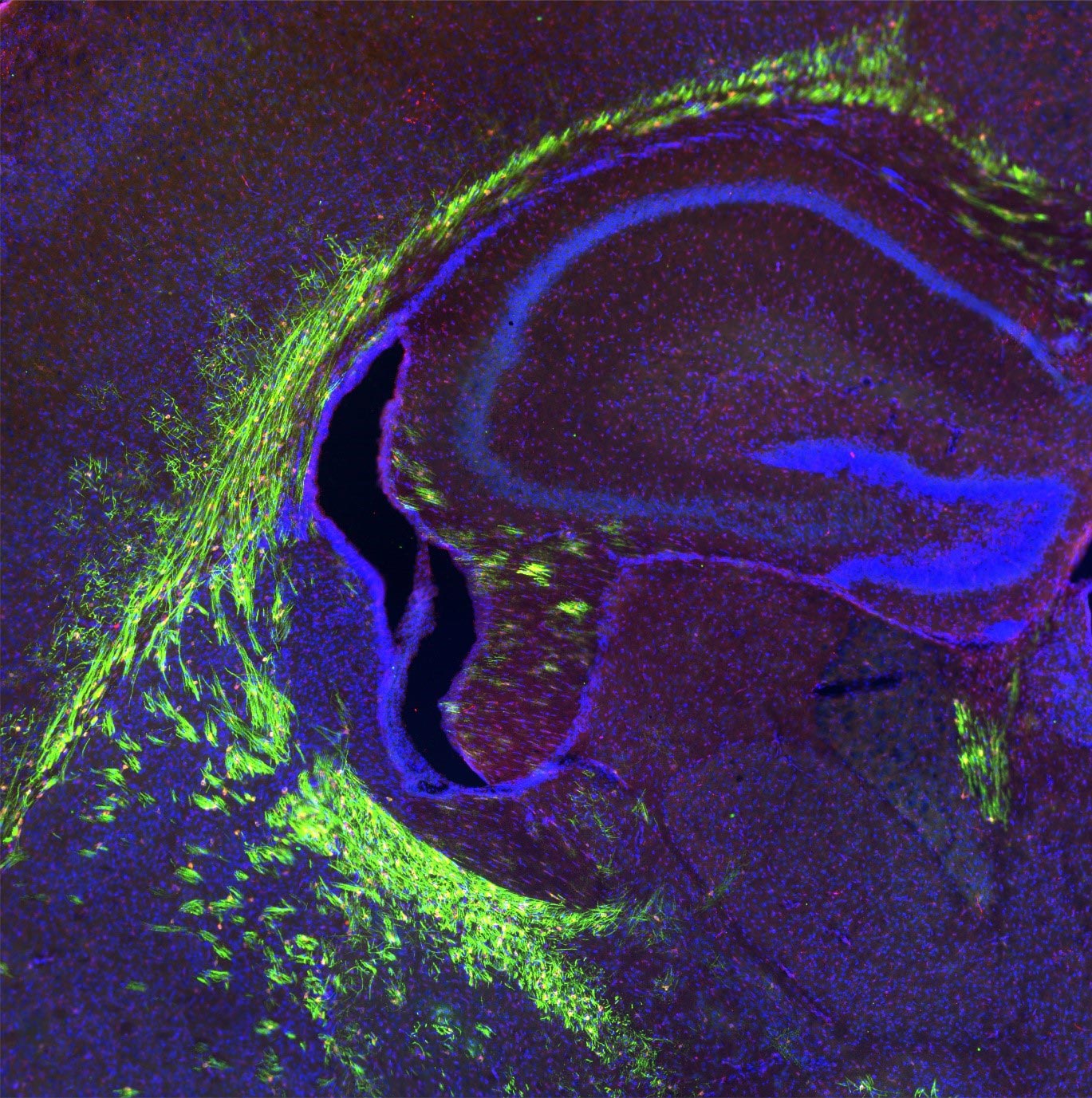
有髄軸索を備えた開発中の前臨床モデルの脳 (緑色で表示)。 シンガポールの科学者は、神経を包む絶縁膜であるミエリン鞘を維持する脳細胞の調節において、トランスポータータンパク質 Mfsd2a の重要な役割を発見しました。 これらの結果は、 ジャーナル・オブ・クリニカル・インベスティゲーション加齢による脳への影響を軽減するのに役立つ可能性があります。 Mfsd2a は、オメガ 3 脂肪酸を含む脂質であるリゾホスファチジルコリン (LPC) を骨髄形成のために脳に輸送します。 クレジット: Vetrivel Sengotuvil 博士
研究者は、Mfsd2a トランスポータータンパク質が、神経を保護するミエリン鞘を維持する脳細胞の調節に不可欠であることを発見しました。 この発見は、老化が脳に及ぼす影響を軽減するのに役立ち、骨髄形成の減少によって引き起こされる神経障害の治療につながる可能性があります。
シンガポールの科学者は、ミエリン鞘と呼ばれる覆いによって神経が確実に保護される脳細胞の調節において、特別なトランスポータータンパク質が果たす重要な役割を示しました. デューク-NUS メディカル スクールとシンガポール国立大学の研究者によって発表された調査結果 ジャーナル・オブ・クリニカル・インベスティゲーション加齢による脳への悪影響を軽減するのに役立ちます。
神経を取り囲む絶縁膜であるミエリン鞘は、体の神経系全体で電気信号の迅速かつ効率的な伝導を促進します。 ミエリン鞘が損傷すると、神経が機能しなくなり、神経障害を引き起こす可能性があります。 年齢とともに、ミエリン鞘は自然に劣化し始めることがあります。これが、高齢者が身体的および精神的能力を失う理由です.
ミエリン鞘の喪失は、通常の加齢過程や、多発性硬化症や神経変性疾患などの神経変性疾患で発生します。[{” attribute=””>Alzheimer’s disease,” said Dr. Sengottuvel Vetrivel, Senior Research Fellow with Duke-NUS’ Cardiovascular & Metabolic Disorders (CVMD) Program and lead investigator of the study. “Developing therapies to improve myelination—the formation of the myelin sheath—in aging and disease is of great importance to ease any difficulties caused by declining myelination.”
To pave the way for developing such therapies, the researchers sought to understand the role of Mfsd2a, a protein that transports lysophosphatidylcholine (LPC)—a lipid that contains an omega-3 fatty acid—into the brain as part of the myelination process. From what is known, genetic defects in the Mfsd2a gene leads to significantly reduced myelination and a birth defect called microcephaly, which causes the baby’s head to be much smaller than it should be.

Dr. Sengottuvel Vetrivel (left) and Prof David Silver (right). Credit: Duke-NUS Medical School
In preclinical models, the team showed that removing Mfsd2a from precursor cells that mature into myelin-producing cells—known as oligodendrocytes—in the brain led to deficient myelination after birth. Further investigations, including single-cell RNA sequencing, demonstrated that Mfsd2a’s absence caused the pool of fatty acid molecules—particularly omega-3 fats—to be reduced in the precursor cells, preventing these cells from maturing into oligodendrocytes that produce myelin.
“Our study indicates that LPC omega-3 lipids act as factors within the brain to direct oligodendrocyte development, a process that is critical for brain myelination,” explained Professor David Silver, the senior author of the study and Deputy Director of the CVMD Program. “This opens up potential avenues to develop therapies and dietary supplements based on LPC omega-3 lipids that might help retain myelin in the aging brain—and possibly to treat patients with neurological disorders stemming from reduced myelination.”
Previously, Prof Silver and his lab discovered Mfsd2a and worked closely with other teams to determine the function of LPC lipids in the brain and other organs. The current research provides further insights into the importance of lipid transport for oligodendrocyte precursor cell development.
“We’re now aiming to conduct preclinical studies to determine if dietary LPC omega-3 can help to re-myelinate damaged axons in the brain,” added Prof Silver. “Our hope is that supplements containing these fats can help to maintain—or even improve—brain myelination and cognitive function during aging.”
“Prof Silver has been relentless in investigating the far-reaching role of Msdf2a ever since he discovered this important lipid transport protein, alluding to the many possible ways of treating not only the aging brain but also other organs in which the protein plays a role,” said Professor Patrick Casey, Senior-Vice Dean for Research. “It’s exciting to watch Prof Silver and his team shape our understanding of the roles that these specialized lipids play through their many discoveries.”
Reference: “Deficiency in the omega-3 lysolipid transporter Mfsd2a leads to aberrant oligodendrocyte lineage development and hypomyelination” by Vetrivel Sengottuvel, Monalisa Hota, Jeongah Oh, Dwight L. Galam, Bernice H. Wong, Markus R. Wenk, Sujoy Ghosh, Federico Torta and David L. Silver, 27 April 2023, The Journal of Clinical Investigation.
DOI: 10.1172/JCI164118

「音楽マニア。プロの問題解決者。読者。受賞歴のあるテレビ忍者。」


More Stories
週末の睡眠を補うことで心臓病のリスクが5分の1減少する可能性がある――研究 |心臓病
化石によると、先史時代のカイギュウはワニとサメに食べられた
二つの大陸で同一の恐竜の足跡を発見